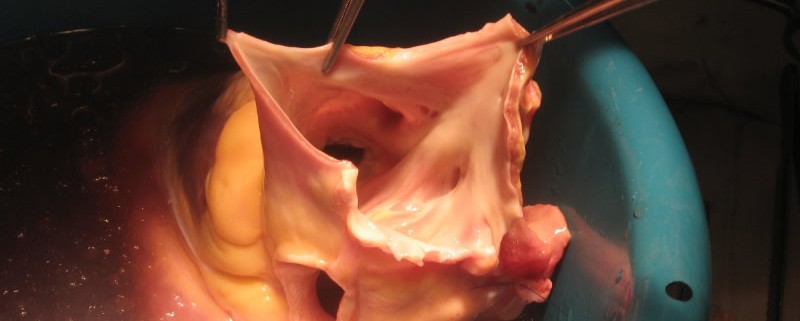
Founded in 1973, medical device manufacturer DeRoyal is committed to improving both the clinical quality and economic health of its customers. With 2,000 employees, 2.5 million square feet (~232,000 square meters) under roof, operations in six countries and five U.S. states, and manufacturing assets on three continents, DeRoyal brings value to its customers in several distinct markets; these include surgical devices, unitized delivery systems, orthopedic supports & bracing, wound care dressings as well as orthopedic implants. Key core competencies include injection molding, device assembly, metal fabrication, converting, electronics assembly, and sterilization services. DeRoyal combines these fundamental manufacturing capabilities with its own unique services and information technology tools to deliver unparalleled value to its customers with a particular expertise in disrupting dysfunctional markets.
For additional DeRoyal product information, please call 1-888-938-7828 within the USA; or +1-865-938-7828 from outside the USA. Visit the DeRoyal website at www.deroyal.com.