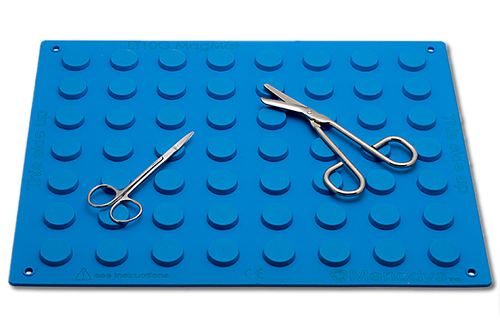
MENODYS’ LT10G Surgical Magnetic Mat is granted a Canadian patent
Longueuil, Quebec, Canada – MENODYS announces that the LT10G Surgical Magnetic Mat was awarded a Grant status from the Canadian Intellectual Property Office on October 16, 2018 (Patent 2,803,194 Magnetic Drape Reducing Magnetic Interferences eg. in surgical applications).
The LT10G was the first medical technology developed and commercialized by MENODYS. Co-invented by Dr LP Fortier and Dr. V. Zaphiratos of the anaesthesiology department of the Maisonneuve-Rosemont Hospital in Montreal, Quebec, Canada, the LT10G is an innovative surgical magnetic drape that reduces the risks of magnetic interferences for patients with implanted electronic devices going through a surgical procedure.
MENODYS is a Canadian company specialized in the research, development and commercialization of medical technologies. www.menodys.com