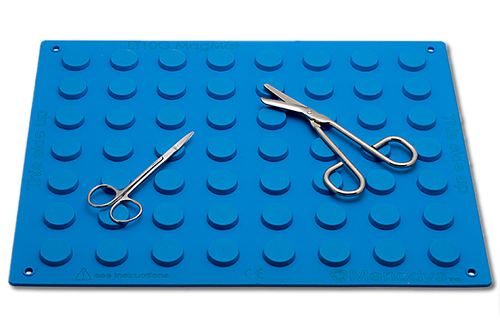
A publication in BioMedical Engineering OnLine demonstrates the safety benefit of using the LT10G Surgical Magnetic Mat of MENODYS in preventing magnetic interferences on a pacemaker in comparison to 4 other commercially available products
Longueuil, Quebec, Canada – Zaphiratos et al. published an article in BioMedical Enginnering OnLine (BioMed Eng OnLine 2016 15:83) entitled: “Interference between surgical drapes and pacemakers: an observational study comparing commercially available devices and a new magnetic isolated drape”, the LT10G Surgical Magnetic Mat from MENODYS. The paper concludes that when the LT10G Surgical Magnetic Mat is used, it does not cause magnetic interference in all patients when placed over the pacemaker. In comparison, three of the four commercially available magnetic drapes tested in the observational study demonstrated magnetic interference on the patient’s pacemaker.
“In an era where implantation of electronic devices is on the rise, surgeons, OR nurses, hospitals and patients should be aware of the potential magnetic interferences caused by surgical magnetic drapes. This study thus confirms that the LT10G offers an important safety benefit in comparison to other available magnetic drapes” commented Richard Côté, EVP of MENODYS.
MENODYS is a Canadian company specialized in the research, development and commercialization of medical technologies. www.menodys.com